CBS News Live
CBS News Texas: Local News, Weather & More
Watch CBS News





Classes at James Bowie High School are canceled for Thursday.

The "all in" comment has been mis-interpreted by some fans and media members, who became critical of Jones for not being "all in" on the free agent market this spring.

A few isolated showers and storms are possible, but most areas will remain dry for much of today.

We've spent long hours filling up our Big Green NFL Draft Scouting Notebook searching for the perfect draft prospects that can propel the Dallas Cowboys to greener pastures.

An already heated campaign over an upcoming school bond referendum now involves the chairman of the Hood County Republican party being arrested and facing a felony charge.

Parents had to wait hours to pick up their children after a fatal shooting outside of Bowie High School.
Despite her experience, the woman tells CBS News Texas she's still Beverly's number one fan and hopes her story is a warning to others.
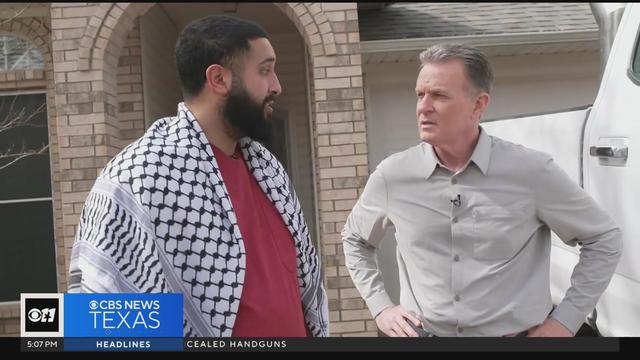
The Arlington resident describes the moment he was stabbed following a pro-Palestine rally at the capitol in Austin.

"These protesters belong in jail. Antisemitism will not be tolerated in Texas. Period."

"If we were to lose control of the Corporation and its assets it would allow the Defendants to remove us from our home, as they have already threatened to do," the Rev. Mother wrote. "I pray they be stopped."

Don Steven McDougal, a family friend, was indicted by a Polk County grand jury in connection with the death of an 11-year-old girl.

Bois D'Arc Lake is now open to families, boaters and fishers just an hour northeast of DFW in Fannin County.
Reggie Bush is getting his Heisman trophy back. The Super Bowl Champion and star running back for USC was stripped of college football's highest honor in 2010. But now, student-athletes can receive compensation for their name, image and likeness.

The Federal Trade Commission is sending refunds to thousands of Ring users after a settlement. The company allowed employees and contractors to access the cameras. It's also accused of failing to implement security protections to keep hackers out of customer accounts.

New federal rules could mean you get your cash back the next time your flight is delayed, or canceled.

Net neutrality is making a comeback with an FCC vote Thursday, which means internet providers will not be able to speed up or even slow down certain web traffic. Now, new FCC rules ban blocking, throttling, or even disadvantaging certain content, apps or services. They also bar prioritizing them in return for payment.

35 northbound as you're moving out of Waxhachie, heading towards Dallas, is shut down Thursday morning. It's all related to a major crash, possibly involving a motorcycle. What we know right now is that all traffic is being forced off and onto the service road at Sterrett Road, which will cause delays.

A few isolated showers and storms are possible, but most areas will remain dry for much of today.

A few isolated showers and storms are possible Thursday, but most areas will remain dry for much of this day.

Isolated showers will be possible Thursday in North Texas but the CBS News Texas weather team expects the cap to remain in place.

A scammer a North Texas woman met on Instagram claimed to be a German cardiologist, and for months, the two messaged back and forth, building what she thought was a true relationship.

Texas police departments have the discretion to determine the frequency and extent of additional driving training for their officers. While some require driving training yearly or every other year, others do not.

Some departments opt to melt the firearms down, while others choose to crush them. However, there are instances where firearms, or at least parts of them, escape destruction altogether.

Several police departments told the CBS News Texas I-Team they were unaware of this practice, even though it was stated in the contracts they signed with the company, Gulf Coast GunBusters.

It's a complicated process that not everyone qualifies for.

The "all in" comment has been mis-interpreted by some fans and media members, who became critical of Jones for not being "all in" on the free agent market this spring.

We've spent long hours filling up our Big Green NFL Draft Scouting Notebook searching for the perfect draft prospects that can propel the Dallas Cowboys to greener pastures.

The WNBA team will stay in Arlington for two more years before relocating to the Kay Bailey Hutchison Convention Center.

Luka Doncic scored 32 points and the Dallas Mavericks overcame the return of Clippers superstar Kawhi Leonard to beat Los Angeles 96-93 and tie their Western Conference first-round playoff series at a game apiece.

The WNBA team says there's now a waitlist for season tickets.

Classes at James Bowie High School are canceled for Thursday.

The "all in" comment has been mis-interpreted by some fans and media members, who became critical of Jones for not being "all in" on the free agent market this spring.

A few isolated showers and storms are possible, but most areas will remain dry for much of today.

We've spent long hours filling up our Big Green NFL Draft Scouting Notebook searching for the perfect draft prospects that can propel the Dallas Cowboys to greener pastures.

An already heated campaign over an upcoming school bond referendum now involves the chairman of the Hood County Republican party being arrested and facing a felony charge.

A scammer a North Texas woman met on Instagram claimed to be a German cardiologist, and for months, the two messaged back and forth, building what she thought was a true relationship.

They found him guilty – now four jurors are explaining how they were convinced to convict Dr. Raynaldo Ortiz.

Texas police departments have the discretion to determine the frequency and extent of additional driving training for their officers. While some require driving training yearly or every other year, others do not.

Some departments opt to melt the firearms down, while others choose to crush them. However, there are instances where firearms, or at least parts of them, escape destruction altogether.

Several police departments told the CBS News Texas I-Team they were unaware of this practice, even though it was stated in the contracts they signed with the company, Gulf Coast GunBusters.

A Texas grand jury indicted more than 140 migrants on misdemeanor rioting charges over an alleged mass attempt to breach the U.S.-Mexico border, a day after a judge threw out the cases.

Regulators prohibit new noncompetes, which impede millions of U.S. workers from getting a better job.

Starting in September of 2024, the year-long residency will allow new teachers to work with veteran teachers before taking on their own classroom.

President Biden signed a foreign aid package into law that includes a potential ban on TikTok in the U.S. Here's what experts say could happen next.

The bond includes upgrades to city streets, parks and public safety facilities. The largest single ticket item in this year's bond includes $50 million for the newly proposed Dallas Police Department's new training facility.

Self-driving 18-wheelers have longtime truckers worried about their livelihood and others concerned that the technology needs more testing to make sure the public is safe.

McDonald's concept restaurant CosMc's has taken its drink-focused menu to Dallas for its second-ever location.

With the country on the cusp of greeting the return of spring, a warm-weather treat is once again available for free for a limited time only.

Kelli and Michael Regan were looking for a new dog. The breeder they found online asked them to pay with gift cards.

Target, looking for ways to add sales, is relaunching its Target Circle loyalty program including a new paid membership with unlimited free same-day delivery in as little as an hour for orders over $35.

The CDC estimates the U.S. could reach 300 measles cases in 2024 — more than the recent peak two years ago.

Organic option is best when buying certain produce, especially blueberries, nonprofit group says in analysis of chemical residues.

The $872 million most likely excludes any amount UnitedHealth may have paid to hackers in ransom.
More than 20 people have been stricken after getting fake or mishandled injections in homes and spas, feds warn.

George Schappell and sister Lori, of Reading, Pa., were the world's oldest conjoined twins, according to the Guinness Book of World Records.

Within hours of the vote, the U.S. Chamber of Commerce announced it would sue to block the ban. Dallas employment attorney Rogge Dunn predicts employees will ultimately win this battle.

The closure affects both Dom's locations in Chicago, and all 33 Foxtrot stores in Chicago, Texas, and the Washington D.C. area.

Texas law SB 14 prohibits drug and surgical "gender transition" interventions for minors.

The projects are expected to create at least 17,000 construction jobs and 4,500 manufacturing jobs.

After more than 40 years in business, 99 Cents Only Stores, a discount chain, announced on Thursday that it will close all 371 of its locations and cease operations.

The "all in" comment has been mis-interpreted by some fans and media members, who became critical of Jones for not being "all in" on the free agent market this spring.

We've spent long hours filling up our Big Green NFL Draft Scouting Notebook searching for the perfect draft prospects that can propel the Dallas Cowboys to greener pastures.

The WNBA team will stay in Arlington for two more years before relocating to the Kay Bailey Hutchison Convention Center.

Luka Doncic scored 32 points and the Dallas Mavericks overcame the return of Clippers superstar Kawhi Leonard to beat Los Angeles 96-93 and tie their Western Conference first-round playoff series at a game apiece.

The WNBA team says there's now a waitlist for season tickets.

Mary J. Blige, Cher, Foreigner, A Tribe Called Quest, Kool & The Gang, Ozzy Osbourne, Dave Matthews Band and Peter Frampton have been named to the Rock & Roll Hall of Fame.

Taylor Swift broke her own records, Spotify said, and now owns the record for the top three most-streamed albums in a single day.

The singer was found deceased at her home, a representative said.

Anticipation was growing at a fever pitch before Taylor Swift's latest album, "The Tortured Poets Department," dropped at midnight EDT. But it turned out it's actually a double album.

The singers first dated in 2003 and delighted fans when they rekindled their relationship in 2023.

Reggie Bush is getting his Heisman trophy back. The Super Bowl Champion and star running back for USC was stripped of college football's highest honor in 2010. But now, student-athletes can receive compensation for their name, image and likeness.

The Federal Trade Commission is sending refunds to thousands of Ring users after a settlement. The company allowed employees and contractors to access the cameras. It's also accused of failing to implement security protections to keep hackers out of customer accounts.

New federal rules could mean you get your cash back the next time your flight is delayed, or canceled.

Net neutrality is making a comeback with an FCC vote Thursday, which means internet providers will not be able to speed up or even slow down certain web traffic. Now, new FCC rules ban blocking, throttling, or even disadvantaging certain content, apps or services. They also bar prioritizing them in return for payment.

35 northbound as you're moving out of Waxhachie, heading towards Dallas, is shut down Thursday morning. It's all related to a major crash, possibly involving a motorcycle. What we know right now is that all traffic is being forced off and onto the service road at Sterrett Road, which will cause delays.

Dallas artist Roberto Marquez traveled to the Rafah Crossing in Egypt, the U.S. capital and will attend this weekend's statewide protest in Austin.

On Friday, hundreds of thousands of fans gathered outside and all around Globe Life Field in Arlington to celebrate the Texas Rangers historical World Series win!

Babies in the neonatal intensive care unit at several Texas Health hospitals were dressed in creative costumes for Halloween.

Is that the smell of cotton candy, beignets and brisket wafting over Fair Park? It sure is, and we are here for it!

No one puts these dolls back in their boxes. Babies in the neonatal intensive care unit at Texas Health Harris Methodist Hospital Southwest Fort Worth are pretty in pink!