CBS News Live
CBS News Texas: Local News, Weather & More
Watch CBS News




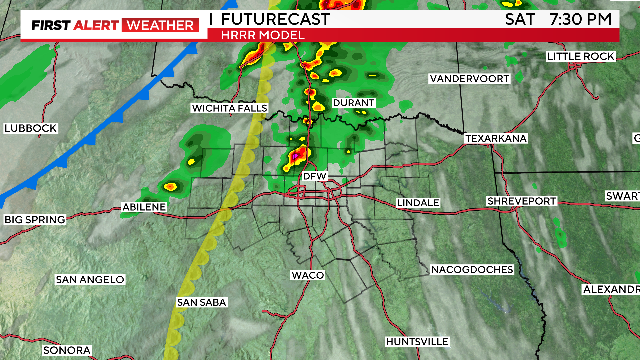
WEATHER ALERTS issued for Saturday afternoon through Sunday afternoon.

The 2024 NFL Draft kicks off Thursday night in Detroit.

Wednesday's shooting outside of the Arlington school devastated current and former students and staff.

Fifty years ago, three-time Indianapolis 500 winner Johnny Rutherford won his first Indy 500, driving a McLaren.

Both Senator Ted Cruz, R-Texas and U.S. Rep. Colin Allred, D-Dallas are running for U.S. Senate this fall, which is the marquee race in Texas.

Each patient celebrated their own superpower and received a cape to wear for the day.

With a relatively low average monthly cost of living and a low crime rate, this little-known town has a lot to offer retirees according to one report.

Border officers have broad authority to search travelers' electronic devices without a warrant or suspicion of a crime.

Angel Gabriel Cuz-Choc was found hiding in a wooded area after his girlfriend and her 4-year-old daughter were found dead in Florida.

"If we were to lose control of the Corporation and its assets it would allow the Defendants to remove us from our home, as they have already threatened to do," the Rev. Mother wrote. "I pray they be stopped."

Don Steven McDougal, a family friend, was indicted by a Polk County grand jury in connection with the death of an 11-year-old girl.

Bois D'Arc Lake is now open to families, boaters and fishers just an hour northeast of DFW in Fannin County.
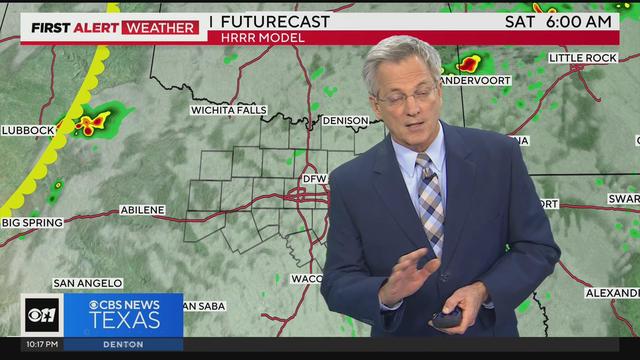
Expect warm and humid conditions for most of the day Saturday as another round of storms return.

Services for a Princeton police officer killed in an off-duty Collin County crash were held Friday.
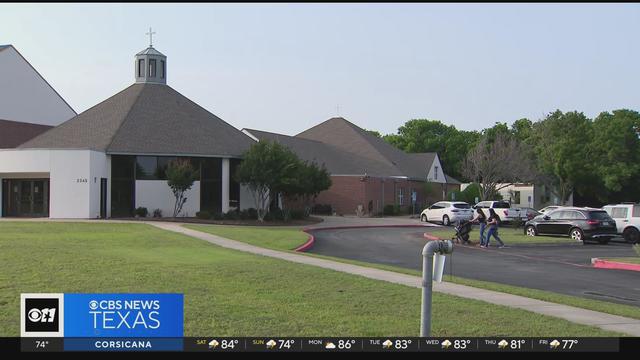
A church held a community prayer service Friday to help those from Arlington Bowie High School deal with a recent fatal shooting.

A race car driver with North Texas roots is celebrating winning his first Indianapolis 500 50 years ago.

Superheroes abounded Friday at Children's Health as the hospital celebrated its annual "Cape Day" as part of National Superhero Day.

Severe weather continues throughout North & Central Texas.
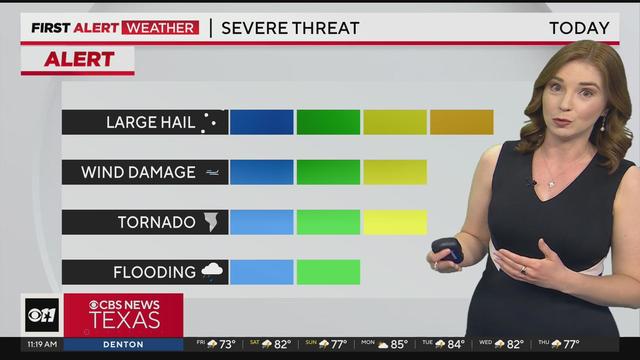
Several North Texas counties are under a tornado watch until 6 p.m.
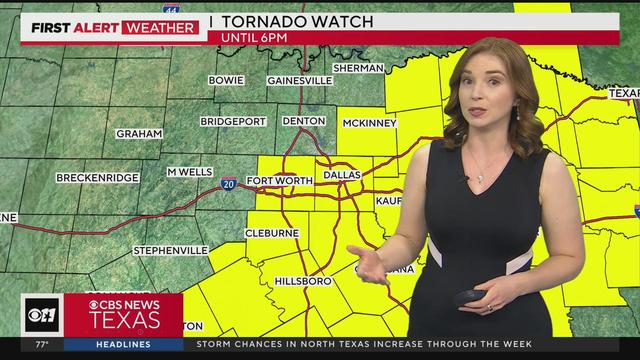
The watch includes Tarrant, Collin and Dallas counties.

A scammer a North Texas woman met on Instagram claimed to be a German cardiologist, and for months, the two messaged back and forth, building what she thought was a true relationship.

Texas police departments have the discretion to determine the frequency and extent of additional driving training for their officers. While some require driving training yearly or every other year, others do not.

Some departments opt to melt the firearms down, while others choose to crush them. However, there are instances where firearms, or at least parts of them, escape destruction altogether.

Several police departments told the CBS News Texas I-Team they were unaware of this practice, even though it was stated in the contracts they signed with the company, Gulf Coast GunBusters.

It's a complicated process that not everyone qualifies for.

With the departure of Tyron Smith, Guyton has massive shoes to fill at left tackle. The starting job will not be given to him, but Jones did not draft him to watch him sit on the bench.

The 2024 NFL Draft kicks off Thursday night in Detroit.

An unprecedented six of the first 12 picks were quarterbacks, an NFL Draft record.

Straight out of the 2024 Big Green NFL Draft Scouting Notebook, here's who the Cowboys won't draft this year, but should.

Noah Hanifin broke a tie with an unassisted goal late in the second period and the Stanley Cup champion Vegas Golden Knights beat the top-seeded Dallas Stars 3-1 on Wednesday night to take a 2-0 lead in the first-round series.

WEATHER ALERTS issued for Saturday afternoon through Sunday afternoon.

The 2024 NFL Draft kicks off Thursday night in Detroit.

Wednesday's shooting outside of the Arlington school devastated current and former students and staff.

Fifty years ago, three-time Indianapolis 500 winner Johnny Rutherford won his first Indy 500, driving a McLaren.

Both Senator Ted Cruz, R-Texas and U.S. Rep. Colin Allred, D-Dallas are running for U.S. Senate this fall, which is the marquee race in Texas.

A scammer a North Texas woman met on Instagram claimed to be a German cardiologist, and for months, the two messaged back and forth, building what she thought was a true relationship.

They found him guilty – now four jurors are explaining how they were convinced to convict Dr. Raynaldo Ortiz.

Texas police departments have the discretion to determine the frequency and extent of additional driving training for their officers. While some require driving training yearly or every other year, others do not.

Some departments opt to melt the firearms down, while others choose to crush them. However, there are instances where firearms, or at least parts of them, escape destruction altogether.

Several police departments told the CBS News Texas I-Team they were unaware of this practice, even though it was stated in the contracts they signed with the company, Gulf Coast GunBusters.

Both Senator Ted Cruz, R-Texas and U.S. Rep. Colin Allred, D-Dallas are running for U.S. Senate this fall, which is the marquee race in Texas.

Border officers have broad authority to search travelers' electronic devices without a warrant or suspicion of a crime.

The Supreme Court convened to consider whether former President Donald Trump is entitled to broad immunity from criminal charges in the 2020 election case.

A Texas grand jury indicted more than 140 migrants on misdemeanor rioting charges over an alleged mass attempt to breach the U.S.-Mexico border, a day after a judge threw out the cases.

Regulators prohibit new noncompetes, which impede millions of U.S. workers from getting a better job.

Self-driving 18-wheelers have longtime truckers worried about their livelihood and others concerned that the technology needs more testing to make sure the public is safe.

McDonald's concept restaurant CosMc's has taken its drink-focused menu to Dallas for its second-ever location.

With the country on the cusp of greeting the return of spring, a warm-weather treat is once again available for free for a limited time only.

Kelli and Michael Regan were looking for a new dog. The breeder they found online asked them to pay with gift cards.

Target, looking for ways to add sales, is relaunching its Target Circle loyalty program including a new paid membership with unlimited free same-day delivery in as little as an hour for orders over $35.

The CDC estimates the U.S. could reach 300 measles cases in 2024 — more than the recent peak two years ago.

Organic option is best when buying certain produce, especially blueberries, nonprofit group says in analysis of chemical residues.

The $872 million most likely excludes any amount UnitedHealth may have paid to hackers in ransom.
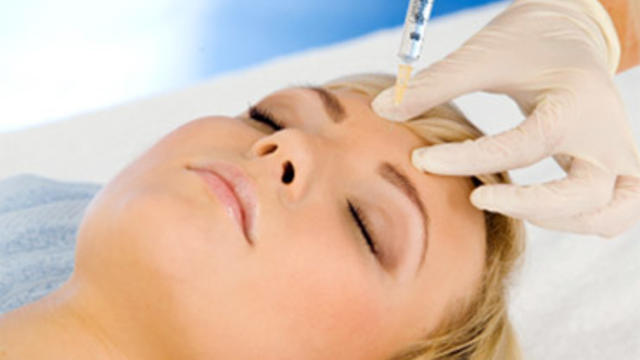
More than 20 people have been stricken after getting fake or mishandled injections in homes and spas, feds warn.

George Schappell and sister Lori, of Reading, Pa., were the world's oldest conjoined twins, according to the Guinness Book of World Records.

Within hours of the vote, the U.S. Chamber of Commerce announced it would sue to block the ban. Dallas employment attorney Rogge Dunn predicts employees will ultimately win this battle.

The closure affects both Dom's locations in Chicago, and all 33 Foxtrot stores in Chicago, Texas, and the Washington D.C. area.

Texas law SB 14 prohibits drug and surgical "gender transition" interventions for minors.

The projects are expected to create at least 17,000 construction jobs and 4,500 manufacturing jobs.

After more than 40 years in business, 99 Cents Only Stores, a discount chain, announced on Thursday that it will close all 371 of its locations and cease operations.

With the departure of Tyron Smith, Guyton has massive shoes to fill at left tackle. The starting job will not be given to him, but Jones did not draft him to watch him sit on the bench.

The 2024 NFL Draft kicks off Thursday night in Detroit.

An unprecedented six of the first 12 picks were quarterbacks, an NFL Draft record.

Straight out of the 2024 Big Green NFL Draft Scouting Notebook, here's who the Cowboys won't draft this year, but should.

Noah Hanifin broke a tie with an unassisted goal late in the second period and the Stanley Cup champion Vegas Golden Knights beat the top-seeded Dallas Stars 3-1 on Wednesday night to take a 2-0 lead in the first-round series.

Harvey Weinstein's 2020 conviction on felony sex crime charges has been overturned by the State of New York Court of Appeals.

Mary J. Blige, Cher, Foreigner, A Tribe Called Quest, Kool & The Gang, Ozzy Osbourne, Dave Matthews Band and Peter Frampton have been named to the Rock & Roll Hall of Fame.

Taylor Swift broke her own records, Spotify said, and now owns the record for the top three most-streamed albums in a single day.

The singer was found deceased at her home, a representative said.

Anticipation was growing at a fever pitch before Taylor Swift's latest album, "The Tortured Poets Department," dropped at midnight EDT. But it turned out it's actually a double album.

Expect warm and humid conditions for most of the day Saturday as another round of storms return.

Services for a Princeton police officer killed in an off-duty Collin County crash were held Friday.

A church held a community prayer service Friday to help those from Arlington Bowie High School deal with a recent fatal shooting.

A race car driver with North Texas roots is celebrating winning his first Indianapolis 500 50 years ago.

Superheroes abounded Friday at Children's Health as the hospital celebrated its annual "Cape Day" as part of National Superhero Day.

Dallas artist Roberto Marquez traveled to the Rafah Crossing in Egypt, the U.S. capital and will attend this weekend's statewide protest in Austin.

On Friday, hundreds of thousands of fans gathered outside and all around Globe Life Field in Arlington to celebrate the Texas Rangers historical World Series win!

Babies in the neonatal intensive care unit at several Texas Health hospitals were dressed in creative costumes for Halloween.

Is that the smell of cotton candy, beignets and brisket wafting over Fair Park? It sure is, and we are here for it!

No one puts these dolls back in their boxes. Babies in the neonatal intensive care unit at Texas Health Harris Methodist Hospital Southwest Fort Worth are pretty in pink!